Fabrication of Novel Polymeric Tissue Scaffolds via Micro-Stereolithography
Project Overview
Goal:
Fabrication of designed tissue scaffolds with controlled porosity, pore size, and pore shape that promote vascularization.
Key Results:
- Fabrication of tissue scaffolds with micron-scale feature sizes from bioactive, biocompatible, and biodegradable materials.
- Understanding the effects of scaffold architecture and mesostructure (e.g. pore size and shape) on cell attachment, viability, and proliferation.
- Fabrication of tissue scaffolds that allow nutrient flow and promote vascularization for the reconstruction of solid tissues.
Description:
The goal of tissue engineering is to combine cells, a tissue scaffold, and combination of electrical, mechanical, or chemical cues to stimulate the repair or regeneration of tissue. Tissue scaffold are structures or templates that promote cell adhesion and the diffusion of nutrients and waste while providing mechanical integrity cells that are seeded on them. Researchers hope that tissue scaffolds can be used to repair or replace damaged or diseased tissue, but current fabrication techniques have not proven adequate for all tissue types, particularly solid vascularized tissue (e.g. kidney, liver, muscle).
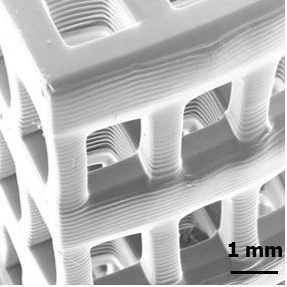
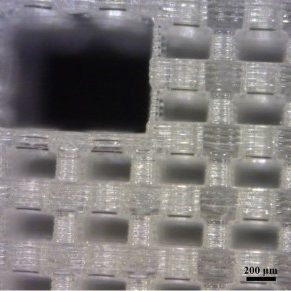
Stereolithography is an Additive Manufacturing process that uses ultraviolet light to selectively polymerize a liquid photopolymer resin layer by layer. These systems are able to achieve higher resolutions than other Additive Manufacturing systems as they are generally limited by the systems optics rather than powder particle size or extrusion nozzle diameter. Micro-Stereolithography is an implementation of Stereolithography that is able to achieve micron-sized features while creating parts with overall dimensions in the centimeter range. This allows for the fabrication of tissue scaffolds with physiologically relevant dimensions.
Unlike conventional tissue scaffold fabrication techniques, Micro-Stereolithography has the ability to control the precise placement of material in a fabricated part. This allows for the simultaneous tuning of pore size, geometry, porosity, and interconnectivity of a tissue scaffold. By tailoring the architecture of a tissue scaffold, cell response such as adhesion, migration, and differentiation can be controlled. In addition, Micro-Stereolithography’s selective material placement has the potential to create tissue scaffolds with vascularization adequate for solid tissues. Tube-like networks can be incorporated into the scaffold design through which nutrients and waste can flow. These networks may also provide the blueprint for cells to create a capillary bed necessary for the vascularization of tissue scaffolds for solid tissue reconstruction.
Although Micro-Stereolithography presents many opportunities for fabricating advanced tissue scaffolds, few materials compatible with the technology are biocompatible, biodegradable, or bioactive. Along with Dr. Timothy E. Long's research group, we are developing and synthesizing novel materials with these properties. Most recently, we have demonstrated the fabrication of tissue scaffolds from poly(tri(ethylene glycol) adipate) dimethacrylate (PTEGA-DMA), a biocompatible and biodegradable photopolymer. Our current work also includes the fabrication of multi-material tissue scaffolds.
Publications
J. Sirrine, A. Pekkanen, A. Nelson, N. Chartrain, C. Williams, T. Long, “3D-Printable Biodegradable Polyester Tissue Scaffolds for Cell Adhesion,“ Australian Journal of Chemistry, 2015, 68 (9), 1409-1414.
P. Lambert, N. Chartrain, A. Schultz, S. Cooke, T. Long, A. Whittington, C. B. Williams, “Mask Projection Microstereolithography of Novel Biocompatible Polymers,” 25th International Solid Freeform Fabrication Symposium, 4-6 August 2014, Austin, TX.


